Discovery of Novel Antifungal Agents Targeting the Synthesis of Glucosyl Ceramide
Invasive fungal infections (IFIs), unlike superficial fungal infections are deep-seated in the tissues. Candida, Aspergillus and Cryptococcus are among some of the most prevalent pathogenic fungal species. While it is quite unlikely for healthy individuals to contract IFIs, immunocompromised individuals are at high risk. Most of the invasive fungal species have displayed a shift towards resistance against currently administered class of drugs, comprising polyenes, azoles, allylamines, echinocandins and flucytosine. Several drugs administered clinically, are known to have a narrow spectrum of activity and some of these drugs are cytotoxic. Identification of novel drug targets and the development of broad-spectrum antifungal agents that combat drug resistance by fungi can help overcome current therapeutic limitations.
Sphingolipids are a class of lipids that participate in cell recognition and tissue
development. In fungi, Glucosylceramide (GlcCer) is critical for cell division to
occur in neutral and alkaline pH. GlcCer is a sphingolipid found in plants, animals,
and fungi. However, the synthetic pathway and molecular structure of fungal GlcCer
differs significantly from mammalian GlcCer (Fig 1). Development of compounds targeting
the fungal GlcCer without affecting the synthesis of mammalian GlcCer is thus an interesting
area to explore.
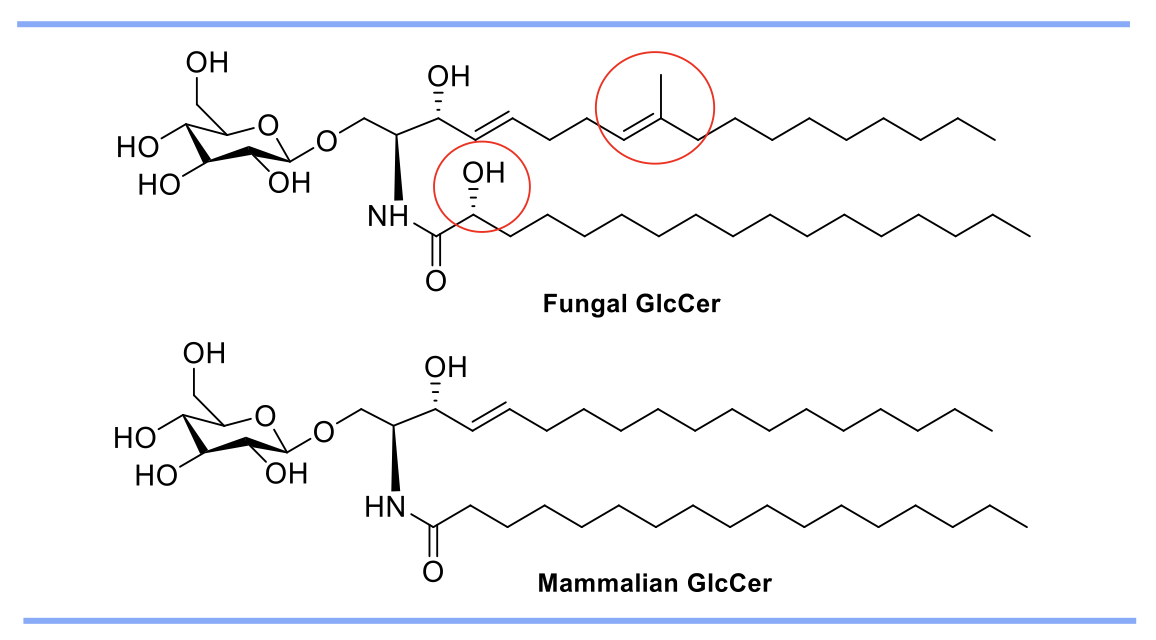
Figure 1. The structures of fungal and mammalian GlcCer
Screening of ~50,000 compounds from ChemBridge library, resulted in five hits. [N'-(3-bromo-4-hydroxybenzylidene)-2-methylbenzohydrazide (BHBM) and its derivative, 4-bromo-N'-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (D2) were highly effective in vitro and in vivo against several pathogenic fungi. D2 possessed high selectivity index and displayed the fungicidal killing activity.
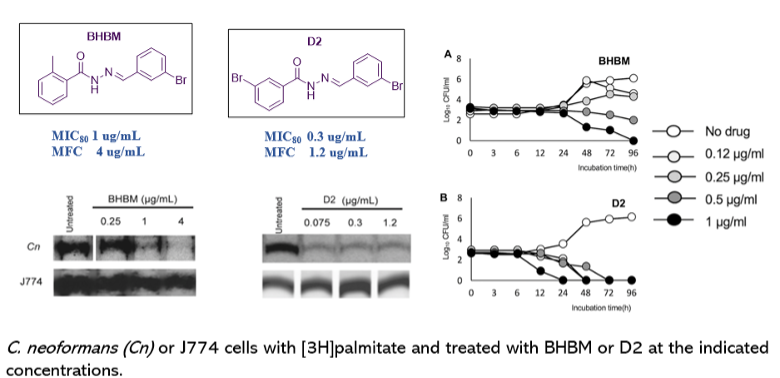
Figure 2. Inhibition of synthesis of fungal GlcCer by BHBM and D2
From studies conducted with similar derivatives, it was observed that aromatic acylhydrazone bearing a salicylaldehyde-hydrazone moiety selectively inhibited the synthesis of fungal GlcCer in a dose-dependent manner without affecting the synthesis of mammalian GlcCer. Based on these results, a new library of 192 acylhydrazones were designed and synthesized and tested against C. neoformans. Out of the 192 compounds, 42 of them displayed MIC80 ≤ 1 μg/mL and, 20 compounds were novel compounds. Based on 20 new compounds' structures, an additional ~350 compounds were synthesized for further exploration of structure activity relationship (SAR) and tested against C. neoformans.
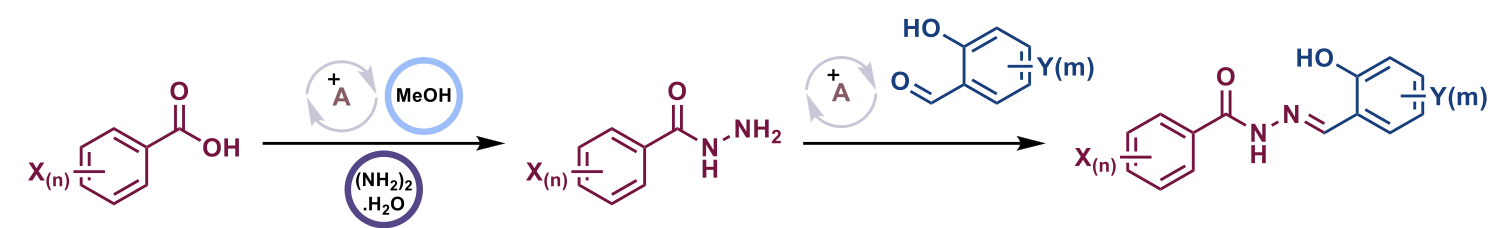
Scheme 1: General synthetic scheme for the synthesis of N′-(Salicylidene)arenecarbohydrazides
The SAR study on the 83 new aromatic acylhydrazones designed, synthesized, and their antifungal activities examined against C. neoformans resulted in several critical findings.
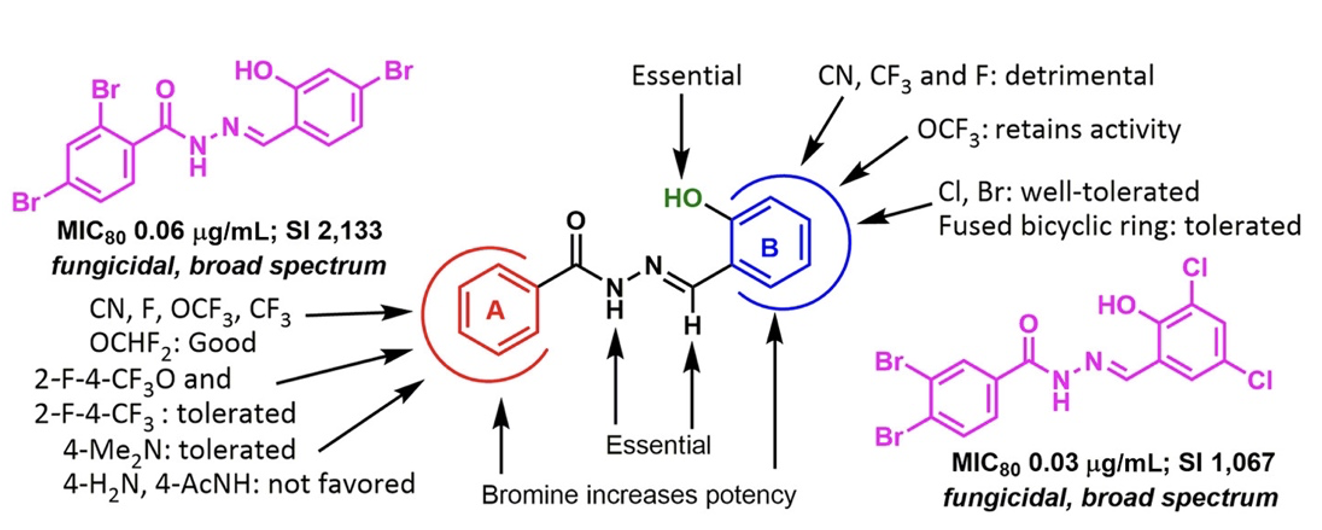
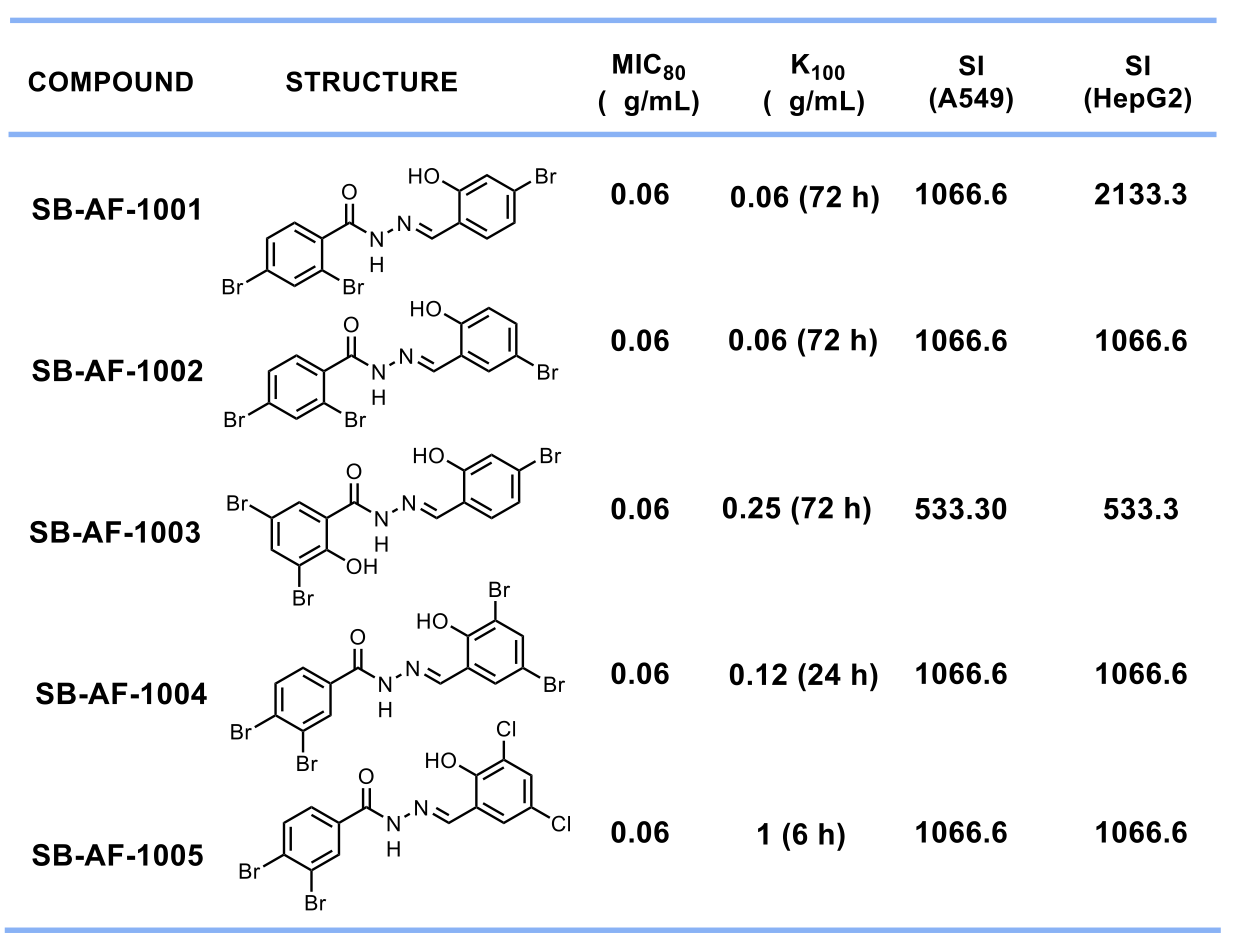
Amongst all the compounds tested, we selected 5 lead compounds that display MIC80 ≤ 1 μg/mL, having fungicidal activity and Selectivity Index > 500. (Table 1)
Table 1. Top 5 lead aromatic acylhydrazones with SI > 500.
Preclinical Evaluation of Acylhydrazone SB-AF-1002 as a Novel Broad-Spectrum Antifungal Agent
Among these top 5 lead compounds, we reported the impressive in vivo activity of 2,4-dibromo-N′-(5-bromo-2-hydroxybenzylidene)benzohydrazide (SB-AF-1002) in several models of systemic fungal infection. Our data displays that SB-AF-1002 is efficacious and outperforms current standard-of-care drugs in models of invasive fungal infections, such as cryptococcosis, candidiasis, and aspergillosis. Specifically, animals treated with SB-AF-1002 not only survived the infection but also showed a clearing of fungal cells from key organs.
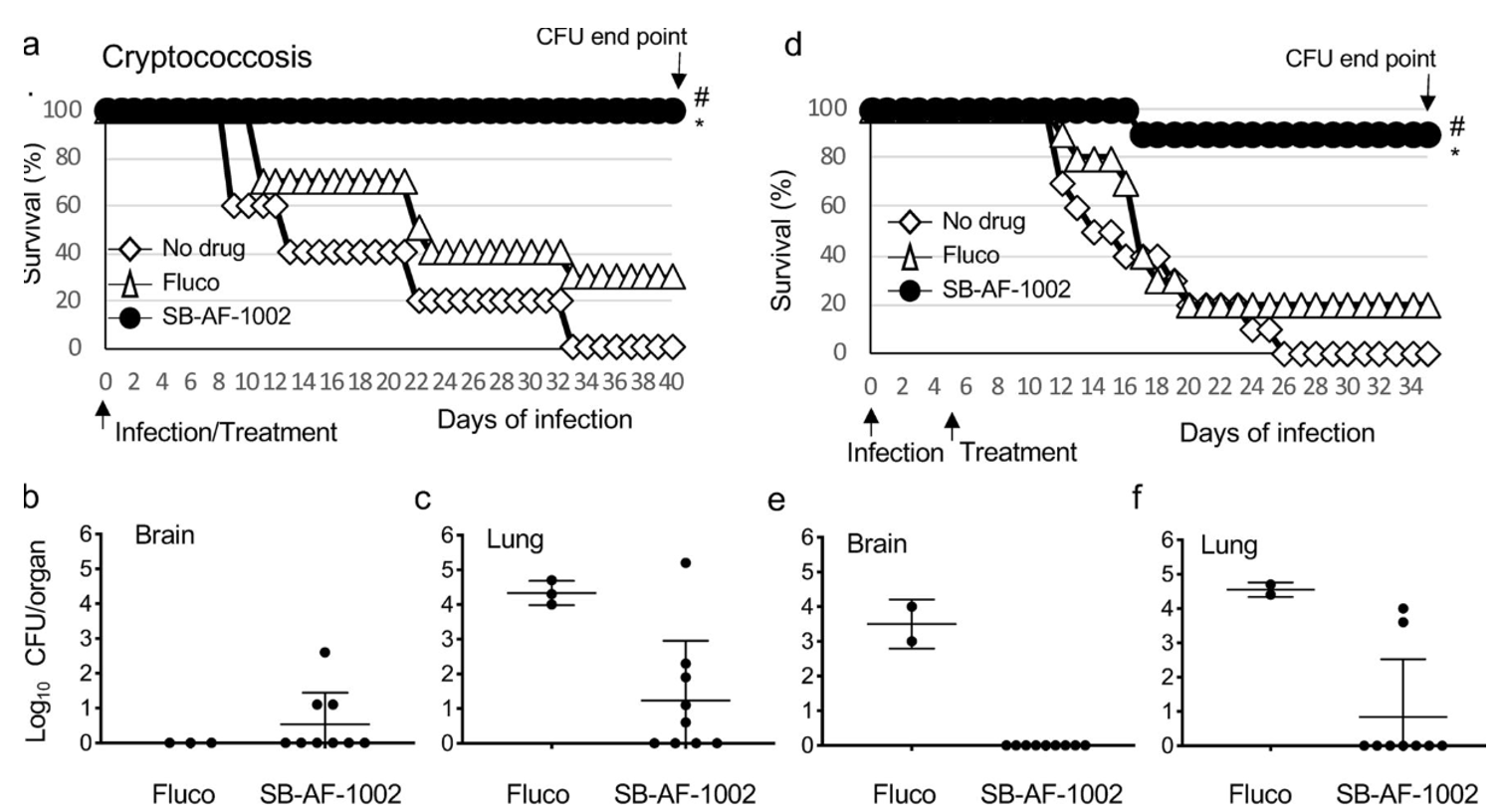
Figure 3. Survival of mice infected intranasally with 5 × 105 C. neoformans cells and treated with 20 mg/kg/day of the compound through gavage. #, P = 0.0001 for SB-AF-1002 versus no drug; *, P = 0.0007 for SB-AF-1002 versus fluconazole (Fluco). (b and c) Endpoint numbers of CFU in the brain and lungs of mice that survived in the experiment whose results are presented in panel a. (d) Survival of mice infected intranasally with 5 × 105 C. neoformans cells with treatment starting at 5 days after infection with 20 mg/kg/day of compounds through gavage. #, P = 0.0001 for SB-AF-1002 versus no drug; *, P = 0.0016 for SB-AF-1002 versus fluconazole. (e and f) Endpoint numbers of CFU in the lungs and brains of mice that survived in the experiment whose results are presented in panel d.
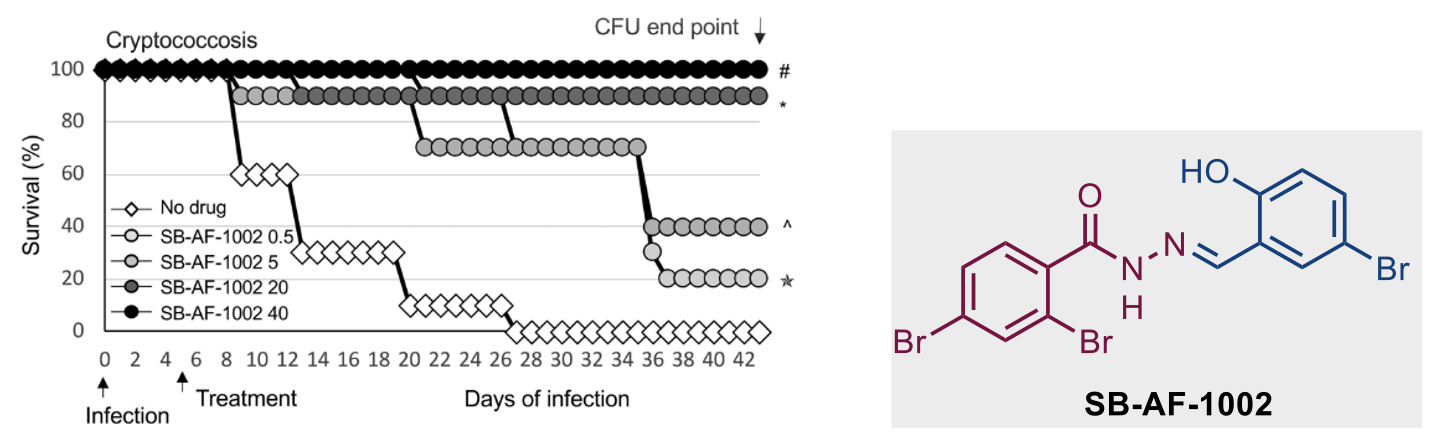
Figure 4. Survival of mice infected intranasally with 5 × 105 C. neoformans cells and treated daily with increasing concentrations of SB-AF-1002 (05, 5, 20, and 40 mg/kg) through gavage, starting at day 5 postinfection.
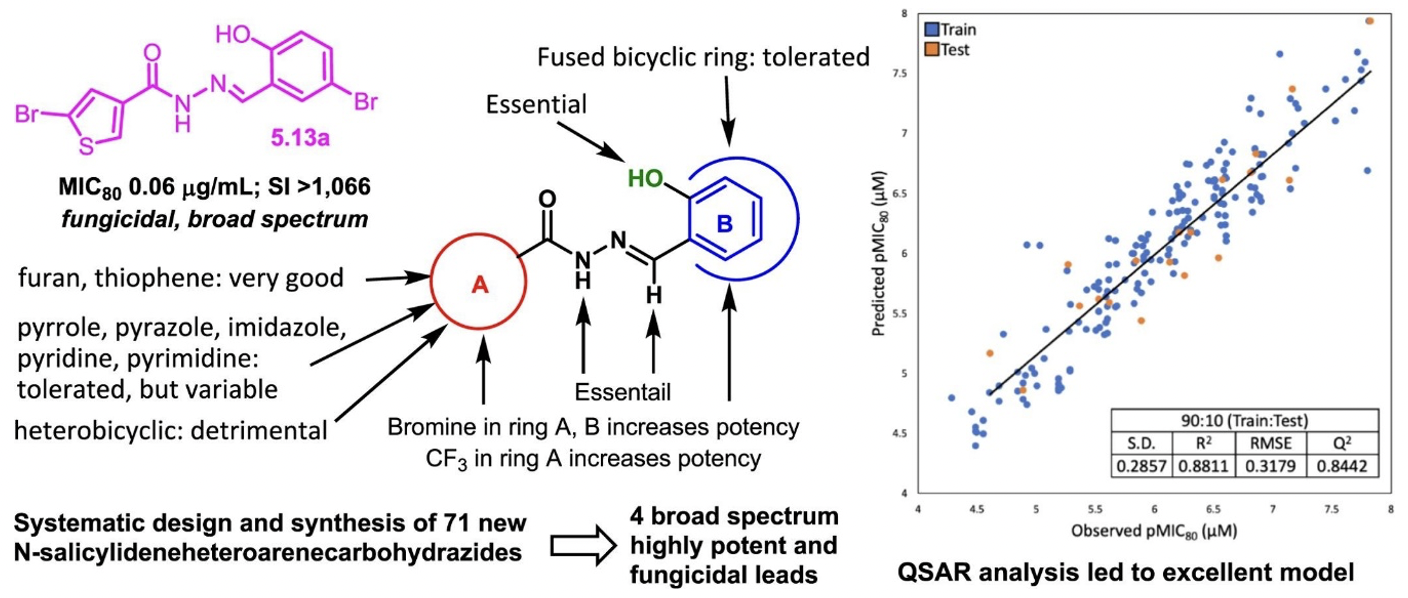
SAR study of N′-(Salicylidene)heteroarenecarbohydrazides as promising antifungal agents.
In a continued effort towards SAR, several heteroaromatic acylhydrazones were synthesized and for this SAR study, we also employed quantitative structure–activity relationship (QSAR) analysis by taking advantage of the accumulated data on a large number of aromatic and heteroaromatic N′-(salicylidene)carbohydrazides, which successfully led to rational design and selection of promising compounds for chemical synthesis and biological evaluation.
Combination therapy of itraconazole and an acylhydrazone derivative (D13) for the treatment of sporotrichosis in cats
Recently, we have also reported the first veterinary clinical study of an acylhydrazone antifungal, 4-bromo-N′-(3,5-dibromo-2-hydroxybenzylidene)-benzohydrazide (D13) combined with itraconazole against a dimorphic fungal infection, sporotrichosis, which is highly endemic in South America in animals and humans. Overall, the results show that the combination treatment was efficacious in ~50% of the infected animals. In addition, D13 was well tolerated during the course of the study. Thus, these results warrant the continuation of the research and development of this new class of antifungals.
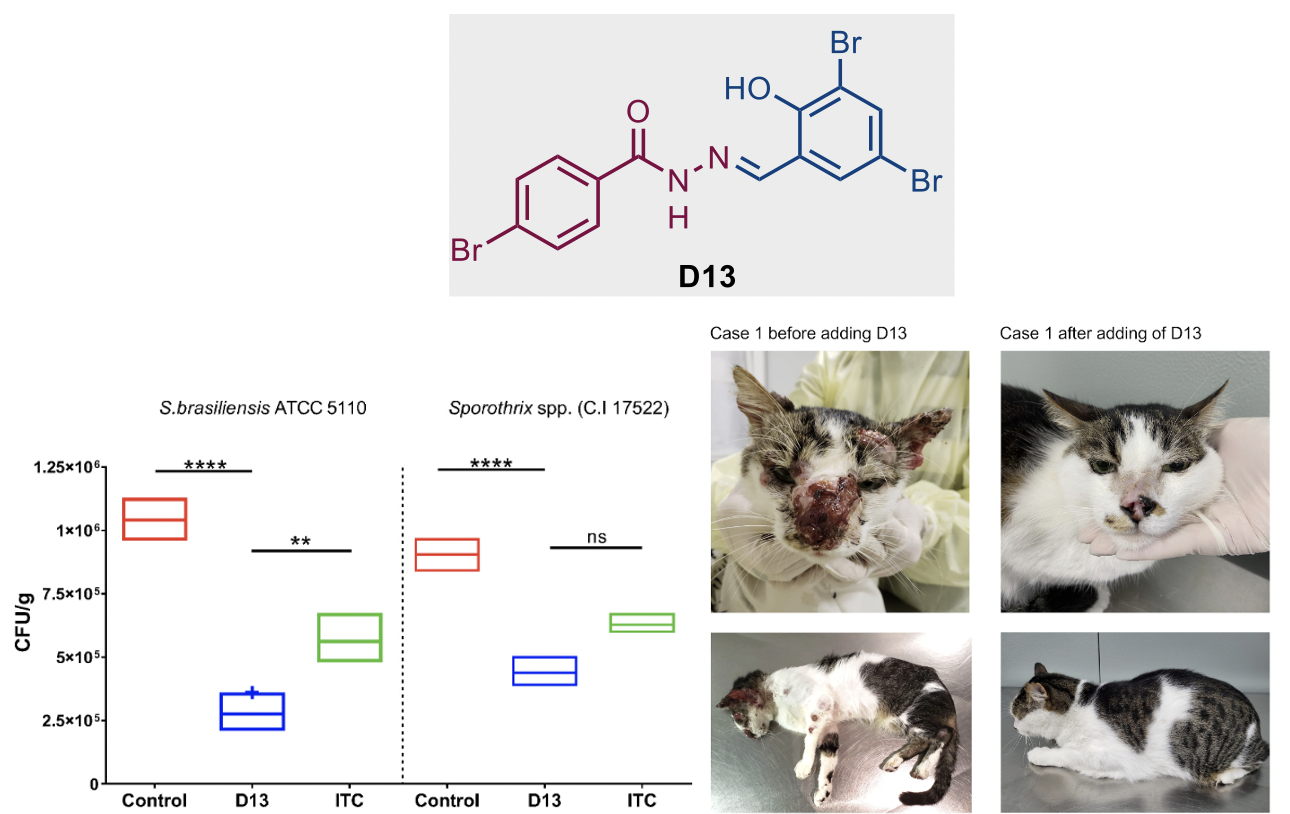
Figure 5. Fungal load of Sporothrix biofilm on cat claw fragments (left). Case 1. The cat presented no improvement of the cutaneous lesions on the head and on the thoracic limbs upon treatment with ITC and KI. After adding D13, the cat significantly improved, and clinical cure was achieved after 8 months of treatment with ITC, KI, and D13 combination (right)
Related Publications
- Mor, V., Rella, A., Farnoud, A.M., Singh, A., Munshi, M., Bryan, A., Naseem, S., Konopka, J.B., Ojima, I., Bullesbach, E. and Ashbaugh, A., 2018. Erratum for Mor et al.,“Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids”. Mbio, 9(2), pp.10-1128.
- Lazzarini, C., Haranahalli, K., Rieger, R., Ananthula, H.K., Desai, P.B., Ashbaugh, A., Linke, M.J., Cushion, M.T., Ruzsicska, B., Haley, J. and Ojima, I., 2018. Acylhydrazones as antifungal agents targeting the synthesis of fungal sphingolipids. Antimicrobial agents and chemotherapy, 62(5), pp.10-1128.
- Mota Fernandes, C., Dasilva, D., Haranahalli, K., McCarthy, J.B., Mallamo, J., Ojima, I. and Del Poeta, M., 2021. The future of antifungal drug therapy: novel compounds and targets. Antimicrobial agents and chemotherapy, 65(2), pp.10-1128.
- Artunduaga Bonilla, J.J., Honorato, L., Haranahalli, K., Dib Ferreira Gremião, I., Pereira, S.A., Guimarães, A., de Souza Baptista, A.R., de Melo Tavares, P., Rodrigues, M.L., Miranda, K. and Ojima, I., 2021. Antifungal activity of Acylhydrazone derivatives against Sporothrix spp. Antimicrobial Agents and Chemotherapy, 65(5), pp.10-1128.
- Haranahalli, K.; Lazzarini, C.; Sun, Y.; Zambito, J.; Pathiranage, S.; McCarthy, J. B.; Mallamo, J.; Del Poeta, M.; Ojima, I. SAR Studies on Aromatic Acylhydrazone-Based Inhibitors of Fungal Sphingolipid Synthesis as Next-Generation Antifungal Agents. J Med Chem2019, 62 (17), 8249–8273.
- Sun, Y., Kim, S., Shin, S., Takemura, K., Matos, G.S., Lazzarini, C., Haranahalli, K., Zambito, J., Garg, A., Del Poeta, M. and Ojima, I., 2024. SAR study of N′-(Salicylidene) heteroarenecarbohydrazides as promising antifungal agents. Bioorganic & Medicinal Chemistry, 100, p.117610.
- Dib Ferreira Gremião, I., Pereira-Oliveira, G.R., Pereira, S.A., Corrêa, M.L., Borba-Santos, L.P., Viçosa, A.L., Garg, A., Haranahalli, K., Dasilva, D., Pereira de Sa, N. and Matos, G.S., 2024. Combination therapy of itraconazole and an acylhydrazone derivative (D13) for the treatment of sporotrichosis in cats. Microbiology Spectrum, pp.e03967-23.